Drug Substance Continuous Manufacturing Working Group
The Drug Substance Continuous Manufacturing Working Group is a collaborative effort to enable the use of continuous manufacturing technology of active pharmaceutical ingredients through addressing the most important areas of interest to Pharmaceutical companies, such as understanding and establishing:
- best practices in the preparation and execution of continuous processes in the current and future regulatory environment,
- current capabilities of Contract Manufacturing Organizations (CMOs),
- advantages of continuous manufacturing when comparing to batch processes
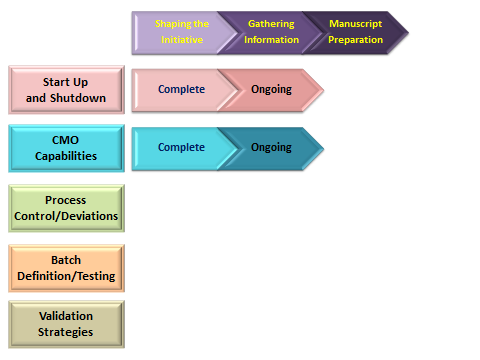
Current Activities
Best Practices in Developing and Executing Continuous Processing to Meet Regulatory Expectations: The Flow Chemistry Working Group is gathering information and working with regulatory agencies to understand all aspects of developing continuous processes in a regulatory environment. The goal of the group is to publish manuscripts focused on specific topic areas that would describe what is known, identify gaps, and propose best practices. Topic areas include:
- Start Up and Shut Down Procedures
- Validation Strategies
- Process Control and Process Deviations
- Batch Definition and Testing
Continuous Processing Capabilities in the External Network: The Flow Chemistry Working Group is developing a survey which will be used to gather information on the knowledge, experience, and continuous processing equipment capabilities of representative Contract Manufacturing Organizations. The information gathered will be shared in the form of a company-blinded publication to enhance overall understanding of the current landscape of capabilities and limitations available in the external network.